Ketogenic Diets
Weight Loss > > Ketogenic diets
Anorectic and Mood-Altering Effects of Ketosis During Ketogenic Diets
Lauri M. Aesoph. N.D. Published in Journal of Naturopathic Medicine
A review of the literature on the anorectic and mood-altering effects of ketosis during carbohydrate restriction in ketogenic diets has revealed inconsistent reports. Early researchers observed that patients reported decreased hunger and elevated mood at the onset of ketosis. More detailed studies, however, found no difference in appetite or emotions between subjects on carbohydrate-restricted and carbohydrate-containing diets. Several theories are offered to explain these initial results, including: 1) subjects' inability to distinguish between hunger, appetite and satiety; 2) diminished food cues during restricted intake; 3) the ability of dietary protein to control satiety through cholecystokinin (when eating a high protein diet) and 4) slow digestion of protein. Mood may appear enhanced because of the control a patient feels while dieting, as well as societal and self approval of weight loss.
Key words: ketosis, ketogenic diet, anorexia, euphoria.
Introduction
Obesity has been treated with carbohydrate restrictive, very low calorie diets (VLCD), particularly protein sparing modified fasts (PSMF) and high fat, high calorie diets. Several reports have drawn a link between ketogenic diets, anorexia and euphoria. It has been suggested that both diminished hunger and improved sense of well-being improve patient compliance during dieting, a supposed advantage for ketogenic diets over carbohydrate-containing hypocaloric diets. However other investigators have questioned whether extended periods of ketosis has any bearing on hunger or mood. This article reviews the literature on this phenomenon.
Historical Review of Ketogenic Diets
One of the first reports of the use of fasting as a treatment for obesity was published 75 years ago1 with subsequent studies appearing in medical journals through the 1960's.2-7 In 1930, Evans and Strang, a research group from the West Pennsylvania Hospital in Pittsburgh, developed one of the first very low calorie diets using ordinary foods as the source of nutrients.8,9 Due perhaps to the monotony and time consuming preparation of these foods, this diet faded into medical obscurity until its idea was revived in 1966 by Bollinger.10 Bollinger and his group recognized the potential hazards of "zero-calorie" diets and very low calorie diets which contained inadequate amounts of protein. In a study using twelve grossly obese subjects, this researcher determined that 40 grams of protein in the form of egg albumin minimized the loss of nitrogen while maintaining anorexia. In 1973, Blackburn described the use of supplemental protein during limited dietary intake as protein sparing therapy,11 also known as a protein sparing modified fast.
It was this pioneering work that ultimately led to a liquid protein diet promoted by Linn in his book The Last Chance Diet.12 The protein used in this weight loss program, composed of collagen, cowhides and hydrolysed gelatin, led to at least fifty deaths probably due to its poor biological quality.14,22 The majority of these deaths have been attributed to ventricular fibrillation.13, 14 Various autopsies have revealed myofibril fragmentation, reduced myocardial fibers and myocarditis. One research group tagged lactic acidosis, with serum ketones topping 20 mg/100 ml, as probable cause of death.l5 These deaths prompted the Food and Drug Administration to publish a warning to the medical community about the potential hazards of using these diets for patients.l6
Since these unfortunate events, manufacturers of protein supplements have revised their products and physicians are now encouraged by the FDA to screen and monitor all patients embarking on a VLCD.14 In addition to vitamins, minerals, and more protein, several protein supplements now include small quantities of carbohydrates. Evidence indicates that ketogenic diets can cause sodium diuresis17,18,l9 fatigue,l8 decreased sympathetic nervous system activity, orthostatic hypotension19 and (in men) gonadotropinuria.20 When subjects were fed enough carbohydrates (45-194 grams/day) to substantially reduce serum ketones, these effects were reversed.l7-20 Carbohydrate consumption while dieting has also been shown to preserve lean body mass and prolong endurance during exercise.21
Proponents of ketogenic diets argue that the ingestion of more than 40 grams of carbohydrates per day abolishes ketosis22 and its supposed anorectic and euphoric effects. Hyperuricemia has been cited as an adverse effect of ketosis.5 While some studies have demonstrated that both fructose23 and sucrose24,25 may also increase serum uric acid, other research has refuted these claims.25,26
The Physiology of Ketosis Healthy, adequately fed individuals on a carbohydrate rich diet produce inconsiderable amounts of ketone bodies.27 When carbohydrate consumption is limited to less than 5 g/100 kcal, as is the case in some ketogenic diets, glucose ceases to be the main source of fuel for the body. Fatty acid oxidation replaces glycolysis as the primary energy manufacturer as elevated levels of acetyl CoA are converted to the ketone bodies acetoacetic acid, beta-hydroxybutyric acid and secondarily acetone in the liver mitochondria. When carbohydrates are reintroduced in sufficient amounts, malonylCoA helps to mediate the inhibition of further ketone body synthesis28 (see Figure 1 ).The blood receives ketone bodies produced by the liver and carries them to the heart29 skeletal muscles30 and brain31 to be utilized as oxidative substrates for fuel. Ketone bodies account for only 10% of the oxygen consumed after an overnight fast.30 However, this figure may increase to between 30% and 90% of oxygen consumption after 48 hours of restricted carbohydrate intake.32 The amount of ketones used is in direct proportion to the arterial concentration up to 70 mg/dl, after which point ketonuria develops.28 Ketogenesis is maximal at three days of total fasting, whereas blood levels peak at three weeks as striated muscles adjust to elevated fatty acid utilization.33
In vitro studies of cardiac muscle and in vivo investigations of striated muscle indicate that free fatty acids (FFA) may compete with ketone bodies for the role of primary fuel source dependent on the serum concentration of each.30 The CNS will accept glucose and/or ketone bodies for energy depending on the length of carbohydrate deprivation and relative concentration of each substrate. During extended ketosis, the CNS may designate up to two-thirds of its fuel substrate to ketones.31 In addition to providing energy, ketones spare protein by reducing the available substrate for gluconeogenesis and subsequently decrease skeletal protein catabolism, as well as decrease the brains's requirement for glucose.34 The contention that ketogenic diets accelerate weight loss when compared to high carbohydrate or mixed hypocaloric diets has been discounted.35
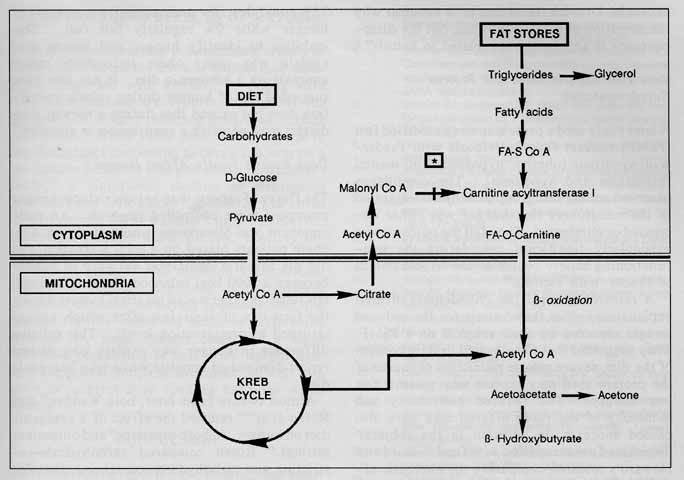
FIGURE 1. How carbohydrates influence triglyceride metabolism. When carbohydrate intake is decreased, malonylCoA is not available to inhibit carnitine acyltransferase I, the enzyme involved in forming FA-O-carnitine (*). This intermediate is able to cross the inner mitochondrial membrane. When acetyl-CoA builds up, as a result of beta-oxidation, ketone bodies are formed (17).
Reported Anorectic Effects of Ketosis
Fasting and Diminished Hunger
In early studies of the use of starvation to treat and control obesity, patients reported a loss of appetite two to four days after their last meal. Anorexia appeared as ketonuria or hyperketonemia developed.3,4,5 Most researchers recognized the value of diminished hunger and its subsequent positive effect on patient compliance. While some investigators merely drew attention to the close relationship between decreased appetite and ketone bodies in the urine or blood,36 Duncan et al concluded that anorexia was most likely due to ketosis.22
Not all investigators were convinced that ketosis played a role in the development of anorexia. Drenick stated that it "is not clear why the sensation of hunger subsides, but the disappearance is apparently not related to ketosis".5
Very Low Calorie Diets and Protein Supplementation
A later study used a protein sparing modified fast (PSMF) to treat four individuals with Prader-Willi syndrome (obesity, hypogonadism, mental retardation and hypotonia). The compliance observed among this group of subjects suggested to the researchers that hunger was either decreased or eliminated. They felt the outcome was particularly significant considering the nonfunctioning satiety center of the hypothalamus of Prader-Willi victims.33
A research group from Philadelphia offered explanations other than ketosis for the reduced hunger reported by their subjects on a PSMF. They suggested that the elevated protein content of the diet, severe calorie restriction or source of the protein used may explain why patients felt less hungry. The reduced palatability and monotony of the foods offered may have also caused anorexia, in addition to the subjects' diminished preoccupation with food.37 Baird and Howard's research comparing the anorectic effect of the drug mazindol, versus a placebo with patients on a VLCD found that the latter caused no decline in hunger. However, compliance was similar in both groups. Monotony and unpalatability, not anorexia, were postulated reasons for dietary adherence.38
Hunger Versus Appetite
In order to assess the effect ketogenic diets have on individuals, researchers must rely on patients' subjective reports of hunger. Problems in studies arise when researchers fail to accurately define hunger. In a paper presented by Hollifield and associates, they attempted to differentiate between appetite, hunger and satiety.39 Appetite was defined as the "willingness to eat" and hunger as the "perception of bodily sensations which can be relieved by eating", both of which fit a set of conditions which induce one to eat. Satiety, on the other hand, was a set of conditions that prompts one to cease eating. In a group of eighty subjects, Hollifield discovered that only 25% could describe and recognize symptoms of hunger while 9% regularly felt full. This inability to identify hunger and satiety may explain why many obese individuals report anorexia on a ketogenic diet. It has also been suggested that if hunger during caloric restriction does not exceed that during a normal diet, dieters may perceive a suppression in appetite.40
Does Ketosis Really Affect Hunger? The flurry of reports that ketosis reduces hunger prompted more controlled research. An early opponent was Silverstone whose study on nine obese patients placed on a total starvation diet did not reveal a significant variance in hunger between a 1000 kcal balanced diet and fasting.41 His subjects experienced maximal hunger during the first day of their fast after which hunger returned to prestarvation levels. This relative difference in hunger may explain why dieters report diminished appetite while on a ketogenic diet.
Almost twenty years later, both Wadden37 and Rosen et al40,42 retested the effect of a ketogenic diet on hunger. In both inpatient40 and outpatient settings,42 Rosen compared carbohydrate-restricting and carbohydrate-containing diets for appetite and mood altering effects. Both studies indicated no significant difference in hunger between the two diets. In accordance with Silverstone's observations, Rosen's inpatient population experienced an increase in hunger during the first week of dieting with a subsequent drop to predieting levels. Wadden's six month study comparing subjects on a 500 kcal PSMF and 1200 kcal balanced diet concurred with Rosen's findings.
Rosen concluded that the impression of anorexia may be due to the relative change in hunger experienced during the first and second weeks of dieting. Other explanations offered by Rosen's group for reported appetite declines were the slow digestion and increased cholecystokinin (a hormone which controls satiety) seen in high protein diets. Both Wadden and Rosen speculated that the paucity of food cues, predictable meals and bland food, especially in a hospitalized setting, could decrease the desire to eat. Rosen disproved his latter theory with his outpatient research.42
Mood Altering Effects of Ketosis Several studies assessing the metabolic effects of fasting have noted the sense of euphoria or well-being associated with ketosis.3,4,10 Along with these observations are reports throughout the literature of weight reducing programs causing depression.43 While there was no difference in psychological functioning between subjects on a low calorie balanced diet and PMSF in Wadden's study,37 a significant decline in depression (p<0.001) and trait anxiety (p<0.002) was noted in both groups. All of Wadden's subjects received behavioral therapy while dieting, possibly re-sponsible for the emotional improvement.
Rosen's study40,42 did not find a significant difference between pre- and post-treatment emotions. He did, however, speculate that the reasons for the improved sense of well-being reported by some dieters could be a more favorable perception of self after weight loss, social approval and reinforcement, as well as a sense of control over feeding behavior while dieting.
Conclusion Early subjective reports have implied that keto-genic diets may improve patient compliance while dieting due to their ability to induce anorexia and euphoria. More recent, comprehensive studies have disputed these claims. Research now indicates that confusion around definitions of "hunger" and "appetite", the rise and decline of hunger during the first weeks of dieting, slow digestion of protein and decreased food cues may instead be responsible for any perception of anorexia. No significant differ-ence in mood has been found between ketogenic and mixed diets. A more favorable view of the dieter by society and self, as well as a sense of control over eating, may explain any improved sense of well being.
References
1. Folin O., Denis W. On starvation and obesity with special reference to acidosis. J Biol Chem 1915;21:183-92 (from reference #22)
2. Bloom WL. Fasting as an introduction to the treatment of obesity. Metabolism 1959;8:214-20
3. Duncan GG, Jenson WK, Cristofori FC, Schless GL. Intermittent fasts in the correction and control in intrac-table obesity. Am J Med Sci 1963;245:515-20
4 . Duncan GG, Jenson WK, Fraser Rl, Cristofori FC. Correction and control of intractable obesity, practical application of intermittent periods of total fasting. JAMA 1962;181(4):309-12
5. Drenick EJ, Swenseid ME, Blahd WH, Tuttle SG. Pro-longed starvation as a treatment for severe obesity. JAMA 1964; 17:100-5
6. Norbury FB. Contraindications to long term fasting (letter). JAMA 1964; 188:18
7. Thompson TJ, Runci J. Miller V. Treatment of obesity by total fast for up to 249 days. Lancet 1966;2:992-6
8. Evans FA, Strang JM. A departure from the usual methods of treating obesity. AM J Med Sci 1929; 177:339-48
9. Evans FA. treatment of obesity with low calorie diets: report of 121 additional cases. Int Clin 1938;3:19-23 (from reference #22)
11. Blackburn RE, Flatt JP, Clowes GHA, O'Donnell TF, Hensle TE. Protein sparing therapy during periods of starvation with sepsis or trauma. Ann Surg 1973;177(5):588-93
12. Linn R. Stuart SL. The Last Chance Diet. Secaucus: Lyle Stuart, 1976
13. Garnett ES, Barnard DL, Ford J., Goodbody RA, Woode-house MA. Gross fragmentation of cardiac myofibrils after therapeutic starvation for obesity. Lancet 1969;1:914-16
14. Anonymous. Details released on deaths of ten on liquid protein diets. JAMA 1977;238(25):2680
15. Cubberly PT, Polster SA, Schulman CL. Lactic acidosis and death after treatment of obesity by fasting. NEJM 1965;272:628-30
18. Bloom WL, Azar GJ. Similarities of carbohydrate deficiency and fasting. Arch Int Med 1963;112:333-7
19. DeHaven J. Sherwin R. Hendler R. Felig P. Nitrogen and sodium balance in sympathetic nervous system activity in obese subjects treated with a low calorie protein or mixed diet. NEJM 1980;302:477-82
20. Kyung NH, Barkan A, Klibanski A, et al. Effect of carbohydrate supplementation on reproductive hormones during fasting in men. J Clin Endocrin Metab 1985;60:827-35
21. Bogardus C, LaGrange BM, Horton ES, et al. Comparison of carbohydrate-containing and carbohydrate restricted hypocaloric diets in the treatment of obesity. J Clin Invest 1981;68:399-404
22. Howard AN. The historical development, efficacy and safety of very-low calorie diets. Intl J Obesity 1981;5:195-208
23. Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ. Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high amylose starch. Am J Clin Nutr 1989;49:832-9
24. Mesquita MF, Seabra MP, Halpern MJ. Simple carbohydrates in the diet. Am J Clin Nutr.1987;45: 1197-201
25. Reiser S. Effect of dietary sugars on metabolic risk factors associated with heart disease. Nutr Health 1985;3:203-16
26. Osei K, Falko J. Bossetti BM, Holland GC. Metabolic effects of fructose as a natural sweetener in the physi-ologic meals of ambulatory obese patients with type II diabetes. Am J Med 1987;83:249-55
27. Barnes RH, Wick AN. A method for the determination of blood acetone bodies. J Biol Chem 1939;131:413-23
28. Montgomery R. Dryer RL, Conway TW, et al. Biochem-istry: a case oriented approach. 4th Ed St. Louis: The CV Mosby Co.1983
29. Williamson JR, Krebs HA. Acetoacetate a fuel of respi-ration in the perfused rat heart. Biochem J 1961;80:540-7
30. Owen OE, Reichard GA. Human forearm metabolism during progressive starvation. J Clin Invest. 1971;50:1536-45
31. Owen OE, Reichard GA, Boden G. Shuman C. Comparative measurements of glucose, beta-hydroxybu-tyrate, acetoacetate and insulin in blood and cerebro-spinal fluid during starvation. Metabolism 1974;23:7--14
32. Wick AN, Drury DR. The effect of concentration on the rate of utilization of beta hydroxybutyric acid by the rabbit. J Bio Chem 1941;138:129-34
33. Bistrian BR, Blackburn GL, Stanbury JB. Metabolic aspects of a protein sparing modified fast in the dietary management of Prader-Willi obesity. NEJM 1977;296:774-9
34. Sherwin RS, Hendler RG, Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest 1975;55:1382-90
35. Pilkington TRE, Gainsborough H., Rosenoer VM, Carey M. Diet and weight reduction in the obese. Lancet 1960; 1:856-8
36. van Riet HG, Schwartz F. der Kinderen PJ. Metabolic observations during treatment of obese patients by periods of total starvation. Metabolism. 1964;13:291--302
37.Wadden TA, et al. Less food less hunger: reports of appetite and symptoms in a controlled study of a protein-sparing modified fast. Intl J Obesity 1987; 1: 239-49
38. Baird IM, Howard AN. A double-blind trial of mazindol during a very low calorie formula diet. Intl J Obesity 1977;1:271-8
39. Hollingfield G. et al. Effects of prolonged fasting on subsequent food intake in obese humans. South Med J 1964;57:1012-16
40. Rosen JC, et al. Comparison of carbohydrate-contain-ing and carbohydrate-restricted hypocaloric diets in the treatment of obesity: effects on appetite and mood. Amer J Clin Nutr 1982;36:463-69
41. Silverstone JT, Stark JE, Buckle RM. Hunger during total starvation. Lancet 1966;1:1343-44
42. Rosen JC, Gross J. Loew D, et al. Mood and appetite during minimal-carbohydrate and carbohydrate-sup-plemented hypocaloric diets. Amer J Clin Nutr 1985;42:371-79
43. Stunkard AJ, Rush J. Dieting and depression re-exam-ined. Ann Int Med 1974;1:526-33
